August 17, 2022 | AI and NLP, Clinical Data Strategy, Digital Quality Measures (DQM), Digital Quality Transformation, Year Round Prospective HEDIS®
Why 2023 Will Be The Watershed Year for Health Plan Quality Teams Transitioning to Digital Quality
This month we are going to discuss what it takes to stand up a digital quality program. Most health plans are somewhere on this transformative journey, either in the planning stages or starting to experiment with potential models. If you are one of these health plans, we think 2023 is going to be the pivotal year for you.
Why is that? Because it’s looking like the pressure to make this hairpin turn will increase substantially in 2024. This gives you one year to get your hands securely on the steering wheel and start practicing those big muscle movements.
Here are the top six things that can help you get started now, so you aren’t scrambling to catch up when the pressure is on in 2024.

Rebecca Jacobson, MD, MS, FACMI
Co-Founder, CEO, and President
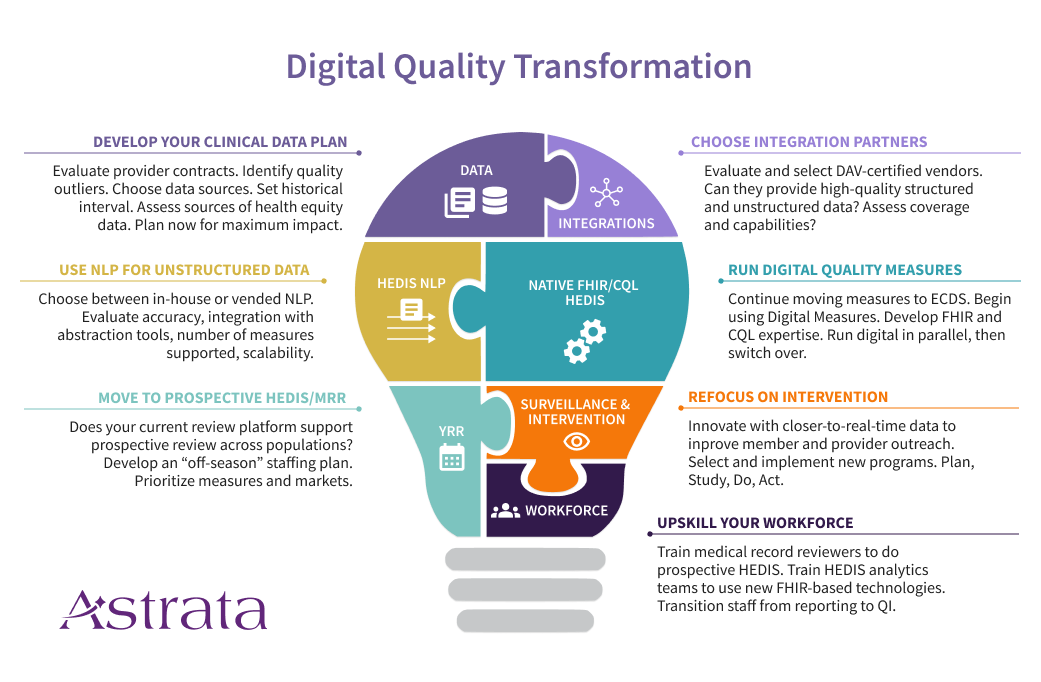
1. Create a Comprehensive Clinical Data Plan
Health Plans are accustomed to measuring quality using administrative (claims) data along with hybrid/sample medical record review. But the future is going to look very different, combining administrative data with clinical data sources such as electronic health record systems (EHRs), care management systems, and health information exchanges (HIEs). You’ve probably started to bring in laboratory and pharmacy data feeds, but that won’t be enough as quality measurement starts to digitize.
That’s because many of the new digital measures will require data elements beyond what you receive in current lab and pharmacy feeds. Consider the strategic changes you’ll need to be successful in this new world of clinical data.
- Do your provider contracts permit data transfer?
- Do you have the infrastructure to support clinical data integration?
- Have you selected one or more data integration partners?
- Are you thinking now about the analytics team you will need (in-house, vended, or both)?
Another critical success factor will be the use of unstructured data along with a high-volume, prospective HEDIS® medical record review. Why? Because better handling of structured data doesn’t mean unstructured data is going away, or that it’s not valuable. Providers will continue to use text fields and clinical notes for documentation. It’s just faster that way. And as a result, the data you need most could be buried in unusable form unless you’re thinking ahead. This is where NLP-powered year-round prospective HEDIS® review becomes essential. When you’re selecting a data integration vendor with NCQA’s Data Aggregation Validator (DAV) certification, remember to also evaluate their ability to provide unstructured data in a format your NLP analytics team or NLP vendor can work with.
Develop your data acquisition strategy and relationships now to support downstream digital quality activities.
2. Leverage Technology
We see two major technology changes on the horizon that will move us closer to the desired end-state. And plans that adopt these technologies earlier are likely going to have an easier time making the transition.
The first major technology change is the use of natural language processing platforms to extract the information needed from unstructured data. NLP has been improving by leaps and bounds over the past ten years. With high-quality data, it’s possible to achieve highly accurate extraction of HEDIS® evidence for current measures. For now, that means working at very fast speeds across populations, with abstractors signing off on cases found by the analytics platform. In the future, we hope to see regulators begin to certify these systems, producing a new and valuable source of standard supplemental data.
The second major technology change is the development of native FHIR/CQL HEDIS® engines. Likely, these new engines will eventually replace the hand-coded HEDIS® engines of yore, for several reasons. First, they will likely be less expensive to create and maintain. Second, they will provide new functionality and speed that current HEDIS® engines cannot provide. Third, the use of native FHIR/CQL will expose intermediate evidence that will be very useful to plans that want to advance their population health initiatives. If your current HEDIS vendor does not have a clear path to transitioning your current measurement, you should start looking for a vendor who does.
Don’t delay in plotting your path to the new technologies. It takes time to find the right vendor partner, educate your team, and manage the change.
3. Manage the Calendar and the Clock
Like many transformational changes, it’s daunting to think through how to maintain your current operations while simultaneously building up your Digital Quality program. You’re building the new plane while you’re still flying the old one. One important thing we are seeing among successful teams is careful management of the calendar and clock.
Your HEDIS® calendar is tight. One of your first steps during the off-season should be moving your medical record review team to a prospective year-round HEDIS® review. As your team comes off sample season in the spring, they should be working from June – January on closing gaps throughout the current measurement year, using pseudo-claims. Initially, you may only be abstracting selected markets or measures in your prospective season. As NCQA removes measures from the hybrid sample, your MRR staff will spend more and more time on prospective review, and less on sample season, until they are doing 100% prospective review. Start that process now, so you can get your tech and dataflows in place and acclimate your team to the differences between these two workflows (and there are a lot of differences).
Managing your clock is just as important. MRR teams have limited time to close gaps. Your abstraction software should be triaging members who are already compliant based on their charts, allowing your team to focus primarily on closing hits and exclusions.
Use the calendar and the clock strategically to gradually transition your team to prospective review, while increasing efficiency to work at population scale.
4. Understand the “New” Data Types and How They Work Together
The future is FHIR (aka HL7 Fast Healthcare Interoperability Resources). HL7 FHIR APIs are the cornerstone for everything we hope to achieve as quality goes digital. The use of FHIR APIs can create a more automated, standards-driven infrastructure for quality measurement. I am particularly excited about the pending federal requirements in the 21st Century Cures Act for EHRs to support bulk FHIR APIs. Effective at the end of 2022, these requirements will enable and facilitate payers’ access to data across populations, potentially at a lower cost than previously possible.
Meanwhile, Data Aggregator Validation (DAV) certified vendors produce standard CCDs from EHRs and HIEs, with the potential to close a lot of gaps. Generally, these files will be most useful for measures where the evidence is already in a structured format. Blood pressures for CBP and HBP, HbA1c results for HBD, and immunization records for PPC are all good examples. If you aren’t already bringing these files in and loading them into your HEDIS® engine, it’s time to start. Meanwhile, you’ll be using your year-round, prospective MRR team to tackle the measures where you really need the unstructured data (e.g. COL, TRC, PPC, etc).
Just keep in mind that soon, digital measures and FHIR APIs are going to change the paradigm. We expect CCD to FHIR mappings to help create an onramp from the current state to the future state. But how exactly this ends up is still a bit of an unknown. So don’t get too attached!
Balance your approach to data by combining structured data (through a DAV-certified vendor) and unstructured data (using an NLP-powered medical record review platform). Recognize that processing CCDs alone won’t help you get at the evidence in unstructured data.
5. Crawl, Walk, Run to Implementation
The adage that every journey begins with a single step is so apt when describing how to move your organization to digital quality. It can seem overwhelming, uncertain, and risky. But it’s better to take the first step early and then adjust. You can start by selecting the markets and measures where you are most worried about the impact of impending changes. These include measures that are soon going to come out of the hybrid sample (like COL) and measures that are moving to digital measurement only (e.g. BCS and CCS). But don’t forget measures where you have the most to gain by intervening earlier (e.g. OMW) and measures where you are typically blind to your final rate (e.g. TRC). These approaches are well-suited to incremental progress and do not require a big bang to get started. Start smaller, learn, and course correct.
Start with markets and measures where you need the help fastest. Show success and then scale.
6. Put All the Pieces Together
Once you have the major pieces in place, you’ve built yourself a platform for quality intervention and innovation. And this is where it is going to get very exciting. It’s likely that as Digital Quality picks up steam, we’ll see entirely new measures developed that are better suited to quality intervention and population health. Instead of a once-a-year reporting frenzy, you’ll be using data during the measurement year, arming your providers with better data to advance your value-based programs, and building intervention programs that have a real impact on the health of your populations. That’s truly what Quality programs were meant to be.
Put all the pieces of the puzzle together to build a quality intervention and innovation platform.
